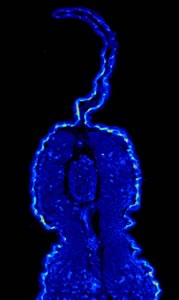
- Fix samplesin
MEMFA and then into Dent's Fix (20% DMSO/80% Methanol)
- If specimens
need to be bleached, bleach in Curis Bleach for
1/2 hour under white light. Store specimens at -20°C in 100%
methanol
- place
embryos into 100% ethanol (dehydrated with molecular sieve (type
3A - BioRad)
- infiltrate
specimens with parafin -- place embryos into glass petri
dish containing molten paraplast (58°-59°C). Repeat with
fresh molten wax.
- fill a
base mold with 5mm parafin -- move embryos into mold with pipette
- 2X washes
with xylene, 15 minutes each
- Add 50%
EtOH and 50% Hemo-De, 20 minutes for large specimens
- Replace
with 100% wax, 2 hours at 58°C
- Replace
with 100% wax, overnight at 58°C *All times are approximate. Large
samples require longer time.
- section
|