 |
Whole-mount & |
E.
coqui stained for ß-catenin |
Whole-mount
immunocytochemistry
developed by Joe
Dent
Whole-mount
immunocytochemistry |
|
|
| Tested in Zebrafish,
Ciona, Coqui, Xenopus and Mouse!
1. Fix specimens for 1 to 2 hours at room temperature with gentle rocking. Typically we use MEMFA(developed in RIchard Harland's lab at UC Berkeley) or Dent's fix (developed by Joe Dent at UC Boulder). MEMFA is better for early stages -- Dent's fixed, early stage xenopus embryos can fall apart, which is quite annoying. 2. If the sample is in MEMFA - replace with Dent's for 15 min. If fixed with Dent's To bleach the embryos - add 2 parts Dents fix and 1part 30% H2O2 and place under bright lamp. Bleach the embryos until they are completely white. At this point, embryos should be transferred to 100% methanol. They can be kept there for quite awhile. |
MEMFA FIX and 10X MEMFA Salts recipes Dent's Fix: 80% methanol and 20% DMSO Dent's Bleach: 1 part 30% H2O2 and 2 parts Dent's fix! |
3. hydrate embryos through TBS/methanol series. wash embryos 3 X in TBS (tris buffered saline) - 15 minutes each washes 4. Incubate overnight in primary antibody diluted into 20% calf serum (our stock of calf serum contains 10% DMSO + 0.05% thimerosal - Sigma T-5152). 5. wash with TBS - once for 15 min., once for 30 min., once for 1 hour, once for 2 hour. |
|
SuperCuris bleach (very fast ) 5% formamide |
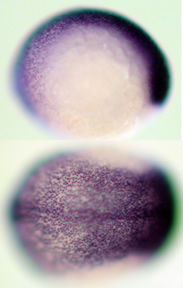 SOX3
in zebrafish SOX3
in zebrafish |
6. Incubate overnight in secondary antibody (diluted into 20% calf serum + 2% DMSO + 0.05% thimerosal). Typically we use peroxidase-conjugate antibodies diluted 1:200 to 1:500 and alkaline phosphatase-conjugated antibodies at 1:2000 to 1:5000. 7. wash as in step 5. - if you are using an alk. phos. conjugated secondary, wash embryos with reaction buffer once (5 to 15 minutes ). 8. react peroxidase-stained embryos with diaminobenzidine reaction solution; react alk. phos.-stained embryos with NBT/BCIP reaction solution . 9.
wash with 100% methanol -- the dehydrated embryos can be cleared in
100% methyl salicylate or BABB: 1 part benzyl alcohol and 2 parts benzyl
benzoate 10.
mount in either a deep depression glass slide or a brass slide |
|
|
| HRP
reaction buffer:
TBS 500µg/ml diaminobenzidine (diluted from a 10 mg/ml stock solution made up in water). 0.02% hydrogen peroxide (diluted from a 30% stock solution). |
AP
buffer:
100mM Tris - pH 9.5 100mM NaCl 50mM MgCl2 0.1% Tween-20 5mM levamisol (can be omitted) |
|
|
|
AP
reaction solution:
NBT diluted from 75 µg/ml in 70% dimethyl formamide |
HRP reaction is complete within 1 hour. AP reaction will continue for hours. |
Plate your cells onto the coverslip and then let them grow until they are at the stage you want. When they are ready, remove the coverslip from the your cells onto the coverslip and then let them grow until they are at the stage you want. Remove the coverslips from the culture (you can keep the culture sterile if you are careful and you flame your forceps). Rinse each coverslip briefly in Tris Buffered Saline (TBS) or Phosphate Buffered Saline (PBS). Place onto staining rack -- normally we use an inverted plastic box, with small corks glued to the top. Coverslips are placed on the corks. |
| Fix the coverslips, typically I generally use 100% methanol for 10 minutes. Wash the coverslip three times by dipping and wisking away excess liquid, into TBS. Incubate with primary antibody (generally diluted into TBS + 0.5% Tween) for 20-25 minutes at room temperature. A typical coverslip requires ~40 µL antibody solution. Wash the coverslip three times by dipping into TBS. Incubate with secondary antibody (we generally using ALEXA conjugated antibodies) for 20-25 minutes. Wash the coverslip three times by dipping into TBS. |
| Place a drop of mounting media onto a glass slide, and then place the coverslip, cells facing the slide, on top. Press down carefully to flatten. Slide can be examined immediately, or left to dry overnight in the dark. The mounting media sets and seals on its own, their is no need to seal the slides. |
Mounting
Media Once dissolved add aliquot (10ml each) and store at 4°C |
| |
| |
updated 31 September 2009 |