| 10. Water, chemical bonds & macromolecular structure |
|
|
|
Water makes up ~70% of the total mass of living organisms. It is quite likely that life arose in an aqueous environment, although the exact steps involved in going from a non-living to a living system remains the subject of ongoing research |
 |
|
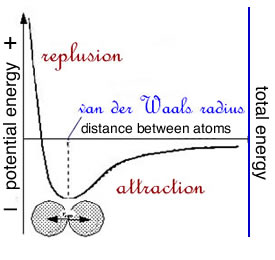
The properties of water arise from the properties of water molecules; they are the major determinant of how cells and their components are organized and how they function. Water is also a reactant/product in many of the reactions that occur in living systems. This means that to understand (i.e. make sense of) biological systems we need to know some basic physics and chemistry, specifically molecular structure, interactions, and thermodynamics [CLUE Chemistry link]. London dispersion forces (a universal glue): All molecules interact with one another through what are known as London dispersion forces (LDF), these are the basis for van der Waals interactions. LDF arise from the weak electrical fields that arise from the movement of negatively (-) charged electrons around positively (+) charged nuclei. These movements lead to a weak and "flickering" electrical field, a temporary dipole, As two molecules approach, these electric fields interact and attract one another. This attraction is strongest when two molecules are separated by what is known as their van der Waals radii. As they get closer, this attraction rapidly turns into an strong repulsion. Each atom and each molecule has its own characteristic van der Waals radius. This is the closest distance two molecules can normally come to another. When we think about the energy of van der Waals interactions, it is important to realize two facts. First according to the laws of thermodynamics, the total energy in the system is constant: energy can not be created nor destroyed, just transformed from one form to another. |
|
While you have probably heard about Einstein's famous equation e (energy) = m (matter/mass) x c2 (where c is the speed of light), energy to matter (and visa versa) conversion does not occur under conditions compatible with life (but rather in the interior of stars, during the explosion of a nuclear weapon, or in gigantic particle colliders). The second fact to keep in mind is that atoms or molecules that bond together always have lower energy thanthe atoms/molecules alone. That is, of course what the word "bond" implies. |
 |
|
Bond stability: When atoms or molecules interact through London dispersion forces, the bond energies are weak. In a solution, molecules are moving rapidly. The temperature of the system is a measure of their Under the conditions where life exists, the kinetic energy of the average molecule is greater than the potential energy of the van der Waals interactions between atoms (and small molecules) - so the bonded atoms (molecules) are knocked apart. Only when there are many van der Waals interactions (as occurs between "macromolecules"(which we will discuss in detail later) is the bonded form stable. |
|
Covalent bonds: In van der Waals interactions, the atoms/molecules retain their hold on their electrons. There are cases, however, where atoms come to "share" each other's electrons. This sharing, known as a covalent bond, produces a much stronger bond interaction (lower energy). link to original chemthink applet |
|
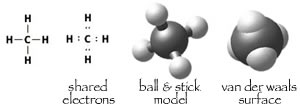 |
When atoms form a bond, their van der Waals surfaces merge to produce a new molecular van der Waals surface. There are a number of ways to draw molecules, but the space-filling or van der Waals surface view is the most realistic (at least for our purposes). |
|
|
|
Polar covalent bonds & enhanced intermolecular interactions: So far, we have been considering covalent bonds in which the sharing of electrons between the atoms involved is more or less equal. The electronegativity of an atom is a measure of how tightly it holds onto its outer orbital electrons. Each type of atom (element) has its own characteristic electronegativity. If the electronegativities of the two atoms in a bond are equal or close, then the electrons are shared more or less equally between them and the bond is said to be non-polar. If the electronegativities of the two bonded atoms are unequal,then the electrons will be shared unequally. |
|
On average, bond electrons will spend more time around the more electronegative atom and less time around the less electronegative atom. This leads to partially negatively and positively-charged sides to the bond. This produces an electrical field, known as a dipole. Such a bond is said to be polar. |
|
looking at this ↑ video, make a list of its inaccuracies. |
|
In biological systems, bonds between O and H, N and H, and C and O are polar - bonds between C and H are "non-polar. In a polar bond, the excess negative charge is associated with the O . The presence of bond dipoles leads to electrostatic interactions between molecules. Since they often involve H bound to O or N, these interactions are known as hydrogen or H-bonds. This is an unfortunate choice of words, since H-bonds are not bonds in the covalent bond sense. They are stronger than van der Waals interactions and have a directionality to them - for an H-bond to form the atoms involved have to be arranged more or less along a straight line. |
|
Questions to answer |
|
|
Questions to ponder
|
|
|
|
|
What are the implications of bond polarity? We can see the consequences of bond polarity by comparing the properties of molecules with and without polar bonds. For example, CH4 (methane) and Ne (neon) have no polar bonds, whereas H2O (water), NH3 (ammonia), and FH (hydrogen fluoride) have three, two and one, respectively. All five have the same number of electrons (10). |
|
Let us now compare the temperatures at which each compound turns from a solid to a liquid (its melting point) and from a liquid to gas (its boiling point). The temperatures at which a compound melts or boils reflects how strongly the molecules of a compound interact with one another. The melting and boiling points of water are high compared to other molecules of similar weight. |
|
|
|
Compounds |
CH4
|
NH3
|
OH2
|
FH
|
Ne |
| molecular weight |
16.04
|
17.02
|
18.02
|
20.01
|
20.18
|
| bond electronegativity |
0.45
|
0.94
|
1.34
|
1.88
|
N/A
|
| # of electrons |
10
|
10
|
10
|
10
|
10
|
| # of bonds |
4
|
3
|
2
|
1
|
0
|
| melting point |
-182°C
|
-77.7°C
|
0°C
|
-83°C
|
-248.6°C
|
| boiling point |
-161.5°C
|
-33.4°C
|
100°C
|
19.5°C
|
-246.1°C
|
|
weird!
|
|
|
|
Because of this arrangement, each water molecule can interact through H-bonds with four other water molecules. To remove a molecule from its neighbors, four H-bonds must be broken.! In the liquid state, molecules jostle one another and change their H-bonded interaction partners constantly. Yet, each water molecule remains linked to multiple neighbors via H-bonds. This molecular hand-holding leads to water's high melting and boiling points as well as its high surface tension. |
|
We can measure the strength of surface tension in various ways. The most obvious is the weight that it can support. Water's surface tension has to be dealt with by all organisms. Some, like the water strider, use it to cruise along the surface of ponds. As the strider walks on the surface of the water, the molecules of its feet do not form H-bonds with water molecules, they are said to be hydrophobic. |
|
Hydrophilic and hydrophobic behaviors: Some molecules, like sugars, alcohols,and most amino acids, dissolve readily in water, they are hydrophilic. Why do some molecules dissolve in water and others do not? Think of water as a network with the links between the molecules formed by H-bonds. To insert a molecule, A, into this network you have to break some of the H-bonds between the water molecules. If theA molecules can make H-bonds with water molecules, then there is little net effect on the free energy of the system. |
|
Such molecules tend to be soluble in water - they are hydrophilic. On the other hand, if A molecules cannot make H-bonds, then when these molecules are inserted into water the total number of H-bonds in the system decreases - the energy of the system increases (remember, bond forming lowers potential energy). However, much of this change in "enthalpy" will be compensated for by van der Waals interactions. Something else must be going on. |
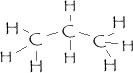 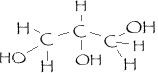 Which molecule is more likely to be water soluble?
|
|
What is happening isthe system responds so as to maximize the number of H-bonds present. This requires that the water molecules become arranged in a shell around each A molecule. Such a shell is more ordered than the normal arrangement of water molecules. This results in a thermodynamically unfavorable decrease in the entropy of the system. |
|
It is primarily because of this decrease in entropy that molecules that cannot make H-bonds with water are relatively insoluble in water - they are hydrophobic. As a general rule, the more polar bonds a molecule contains, the more hydrophilic it will be; the fewer polar bonds, the more hydrophobic. Basically, the more H-bonds it can make the more it "looks" like water. At the same time, the smaller the molecule, the smaller are the effects of inserting it in between water molecules. The solubility of a molecule in water depends upon its size, shape and the number of polar bonds it contains. |
|
|
|
These general rules of molecular interactions with water also apply to macromolecules, such as lipids, carbohydrates, proteins, and nucleic acids, which we will consider in greater detail later. Most macromolecules contain both hydrophilic and hydrophobic groups. The hydrophobic groups will tend to be buried in the center of the molecule (or macromolecular complex), minimizing their interactions with water. Carbohydrates illustrate this rule - they are almost exclusively hydrophilic and tend to adopt an extended structure in solution. In soluble proteins, hydrophilic groups tend to be on the outside, maximizing their interactions with water, while hydrophobic groups tend to be buried in the interior of the molecule (thus minimizing their interactions with water. |
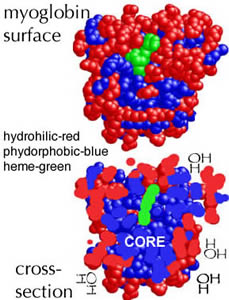 |
|
Because of the polar nature of their bonds, water molecules interact with ions, thereby making them soluble. Take common table salt, NaCl, which normally exists as a crystalline solid. The bonds between the Na and Cl atoms are totally ionic - electrons are not shared, rather Na gives up an electron and becomes Na+, while Cl accepts the electron and becomes Cl-. The solid is held together by electrostatic interactions (ionic bonds). |
|
To remove a Na+ or a Cl- ion from a salt crystal involves separating positive from negative charges. In water, these charges are largely neutralized by interactions with the partially charged groups of the water molecules. As salt dissolves, the Na+ and Cl- ions become surrounded by a shell of oriented water molecules. Water does the same thing to itself. |
|
|
It stabilizes its own dissociation into OH- (hydroxyl) and H+ ions. The H+ ion, a naked proton, does not exist as such, but is associated with water molecules, forming H3O+, H5O2+ and larger ions. In pure water, [OH-] = [H+]and equal 10-7 molar. That means that in a solution of pure water there are 10-7 moles of OH- and 10-7 moles of H+ per liter. |
|
While these are small numbers, small changes in the concentrations of OH- and H+ have important effects in biological systems (see here). You might want to brush up on the meaning of a Molar solution, the definition of pH, and what it means to be an acid or a base. |
|
|
|
|
Questions to answer |
|
|
Questions to ponder
|
|
|
|
| replace with revised beSocratic activity |
|
|
|
Tweet
revised
10-May-2014
|