 |
Both sperm and egg are highly specialized, they are themselves the products of complex cellular differentiation processes. When they fuse, they create a new diploid cell, the zygote, which becomes the embryo and eventually the adult. |
| The division of the zygote, by mitosis, begins the process of embryonic development. |
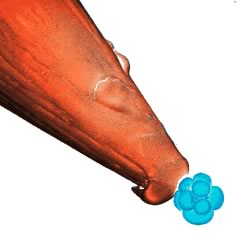 An
8-cell mouse embryo on the head of a pin. An
8-cell mouse embryo on the head of a pin.
|
As the zygote divides, the embryonic cells begin to behave differently from one another. One reason is that each cell comes to have a different environment, different neighbors. |
In the mouse, a major difference is whether cells are on the surface of the embryo (the trophectoderm) or within the interior (inner cell mass). |
mouse embryo references Zernicke-Goetz and
Motosugi et al |
|
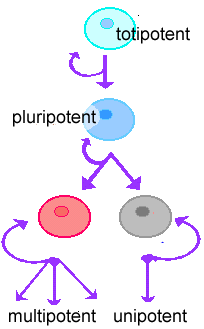
As the zygote divides, the cells it differentiates into form the many different cell types of the fetus and eventually the adult: skin, muscle, nerve, hair, bone, etc. The process of differentiation proceeds in a stepwise fashion and involves changes in gene expression. It is easy to tell a muscle cell from a neuron from a bone cell from a skin cell by the set of genes they express, the proteins they contain, their shape (morphology), their internal organization, and their behavior. The zygote created by fertilization is totipotent, it can generate all of the cells of the adult. As development proceeds, however, cells become more and more restricted with respect to the types of cells that they can produce -- they become committed to one or another specific fate. |
 |
There are two basic, and interacting, processes that drive embryonic development. During the formation of the egg and following fertilization, cytoplasmic determinants can become localized to specific regions of the egg, and later to specific regions of the embryo. The presence of these factors, they may be proteins or RNAs, drives the cell that contains them in a specific developmental direction. |
This developmental direction is based on altered gene expression. The second set of processes involved in embryonic development are the changing interactions between cells. These involve adhesive interactions and intercellular signaling systems. They can direct a cell to adopt specific fates. These processes are the subject of developmental biology and beyond this class. |
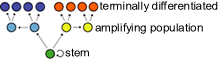 |
Stem cells are cells that continue to divide, and remain undifferentiated. Generally, in each stem cell division, one cell remains a stem cell, while the other goes on to differentiate. |
The stem cells themselves usually divide rather infrequently (and are rather rare). The differentiating daughter, on the other hand, may divide a number of times before it terminally differentiates. These dividing cells are known as the amplifying population. Such cells are committed to differentiation and have a finite proliferative life span - they can divide only a limited number of times before they senesce. Terminally differentiated cells no longer divide. |
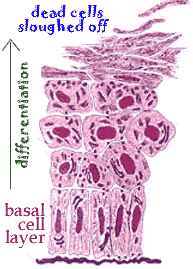
Adult stem cells are responsible for tissue regeneration. For example, your skin is constantly being replaced. This process involves epidermal stem cells, located in your hair follicles. The progeny of these cells move into the basal layer of the epidermis and the matrix of the hair follicle where they continue to divide, differentiate and eventually die. In normal skin the process of cell birth and death is balanced. Hyperplasia occurs when cell birth occurs more frequently than cell death. |
 |
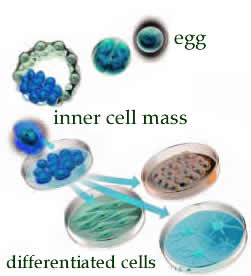 |
During mammalian development, two classes of cells emerge early on. The cells of the trophectoderm form extraembryonic tissues, that is the membranes that surround the embryo and the placenta. The cells of the inner cell mass form the various tissues of the organism – it is these cells that are totipotent. It is the inner cell mass that is used to isolate embryonic stem cells. |
Embryonic stem cells can be cultured in vitro; under certain conditions they can be induced to differentiate into various cell types. Since the generation of totipotent human embryonic stem cells involves the destruction of a human embryo, it raises a number of ethical issues as well as technical problems. More recently, there have been a number of reports that differentiated cells from adults can be "reprogrammed" by the introduction of various actively expressed genes (and other methods), to form cells that behave much like embryonic stem cells. (references here, here and here). |
Cellular differentiation and genomic information: An important question that was asked by early developmental biologists was, is cellular differentiation due to the loss of genetic information Is the genetic complement of a neuron different from a skin cell or muscle cell? This question was first approached by Briggs and King in the 1950s through nuclear transfer experiments in frogs. |
 |
 |
These experiments were extended by Gurdon and McKinnell in the early 1960s. They were able to generate adult frogs via nuclear transfer using embryonic cells. The process was inefficient however - only a small percentage of transferred nuclei supported normal embryonic development. In 1996 Wilmut et al used a similar method to clone the first mammal, Dolly. |
Since then many different species of mammal have been cloned, and there is serious debate about the cloning of humans. In 2004, cloned mice were derived from the nuclei of olfactory neurons using a method similar to that used by Gurdon. These neurons were derived from a genetically engineered mouse that expressed green fluorescent protein (GFP - the gene was isolated from the jellyfish Aequorea victoria). A hybrid gene
that contained the coding sequence for GFP and a regulatory sequence
that led to expression in most cell types was inserted into
the mouse cells. Neuronal nuclei were transplanted into an oocyte from which the original nucleus had been removed (an enucleated oocyte). Blastula derived from these cells were then used to generate totipotent embryonic stem cells. It was the nuclei from these ES cells that were transplanted into enucleated eggs. |
The resulting embryos were able to develop into full grown mice, proving that neuronal nuclei retained the information required to generate a complete adult animal. The process of cloning from somatic cells is inefficient – many tries have to be performed, each using a egg, to generate an apparently normal embryo (most embryos were highly abnormal). Currently research is ongoing on trying to optimize the process by which somatic nuclei can be reprogrammed to support totipotent and pluripotent development. At the same time, there are strong ethical concerns about the entire process of reproductive cloning. |
 |
Questions to answer
|
|
Questions to ponder |
|
| replace revised beSocratic activity |
Tweet
revised
10-May-2014
|