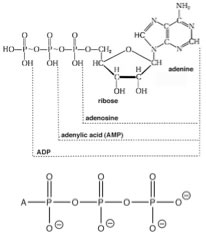
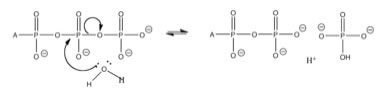
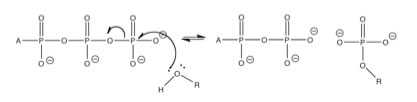
While, previously we have considered single reactions which we have perturbed, either by adding or taking away matter (reactants or products) or energy (heating or cooling the reaction), now it is time to look at what happens when reactions are coupled - that is: the products of one reaction are the starting materials for other reactions, occurring in the same system.
Take for example the coupled system introduced above - the pair
of reactions that are linked by the formation and reaction of
carbonic acid.
H2O + CO2 ↔ H2CO3 and H2CO3 + H2O ↔ HCO3– +
H3O+
These coupled reactions are important for a number of reasons,
including transport of excess carbon dioxide to the lungs, and
for buffering the pH of blood. Carbon dioxide enters the blood
stream by dissolving in the plasma, however it can also react
with water, in a reaction where the water acts as a nucleophile
and the carbon dioxide acts an an electrophile.
| The formation of carbonic acid is thermodynamically unfavorable, the equilibrium constant for hydration of carbon dioxide is 1.7 x 10–3. The standard free energy change for the reaction (ΔGº = –RTlnK), so at physiological temperatures (37º C) ΔGº is 16.4 kJ. |  |
This means that the amount
of carbonic acid in blood plasma is quite low; most carbon dioxide
is just dissolved in the plasma (rather than reacted with the
water). That said, as soon as carbonic acid is formed, it can
react with water
H2CO3 + H2O ↔ HCO3– + H3O+
to produce bicarbonate (HCO3–); the rate of this reaction
is increased by the enzymatic catalyst carbonic anhydrase. In
this buffer system the carbonic acid can react with any base
that enters the bloodstream, and the bicarbonate with any acid.
This buffering system is more complex than the isolated ones
we considered earlier, since one of the components (carbonic
acid) is also part of another equilibrium reaction. In essence,
this means that the pH of the blood is dependent of the amount
of carbon dioxide in the bloodstream.
H2O + CO2 ↔ H2CO3 + H2O
↔ HCO3– + H3O+
If we remove water from the equations (for the sake of clarity)
we can (hopefully) see the connection better.
CO2 + H2CO3 ↔ HCO3– + H3O+
The pKa of carbonic acid is 6.37, and the pH of blood is typically
7.2-7.4, which does fall (just) within the buffering range.
| Under
normal circumstances this buffer system can handle most changes
but for larger changes, other systems are called into play to
help regulate the pH. So for example, if you exert yourself,
one of the products generated is lactic acid, (which we will
denote as LacOH)192. When it finds its way into the bloodstream
it lowers the pH (increasing the amount of H3O+), through the
reaction: LacOH + H2O ↔ H3O+ + LacO– |
 |
If we use Le Chatelier’s principle, you can see that increasing
the H3O+ shifts the equilibrium towards the production
of carbon dioxide in the buffer system. As the concentration
of CO2 increases,
a process known as chemoreception activates nervous systems that
in turn regulate (increase) heart and respiratory rates, which
in turn leads to an increase in the rate of CO2 and
oxygen exchange in the lungs - as you breathe in O2 you
breathe out CO2 (removing
it from your blood). In essence - Le Chatelier’s principle
explains why we pant when we exercise! Conversely, when some
people get excited they breathe too fast (hyperventilate); too
much CO2 is removed from the blood, which reduces
the H3O+ concentration and increases the pH. This
can lead to fainting (which slows down the breathing), a rather
drastic way to return your blood to its correct pH. An alternative,
non-fainting approach is to breathe into a closed container.
Since you are now breathing expelled CO2 (and a lower
level of O2),
this also leads to an increase in blood pH.
While we invoke Le Chatelier’s principle to explain the
effect of rapid or slow breathing, this response is one based
on what are known as adaptive and homeostatic systems. Biological
systems are characterized by many such, often interconnected,
regulatory mechanisms, that have evolved to maintain a stable
chemical internal environment essential for life. Similar coupled
regulatory systems lie at the heart of immune and nervous system
function. Understanding the behavior of these coupled systems
is at the forefront of many research areas. At their base, they
rely on chemical systems to measure the levels of various chemicals
(a process known as chemoreception), recognize and respond to
foreign molecules (in the case of the immune system), and respond
to stimuli from both the outside world (light, sound, smell,
touch) and internal factors (in the case of the nervous system).
Downstream of these ”sensory” systems, are networks
of genes, proteins and other molecules whose interactions are
determined by the thermodynamics of the chemical system. While
they were formed by evolutionary processes, and are often baroque
in their details, they are understandable in terms of the molecular
interactions, chemical reactions, and their accompanying energy
changes.