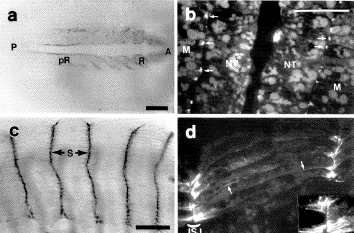
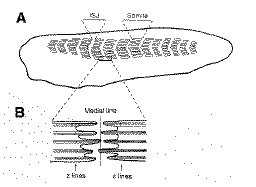
Myotomal muscle
cells span the entire length of each somite, and are connected
to one another at the intersomite junction. This is a cell-extracellular
matrix junction, analogous to the muscle-tendon junction
of later stage animals.
As expected,
disruption of the desmin filament network did not disrupt the
formation of the actomyosin-based contractile apparatus.
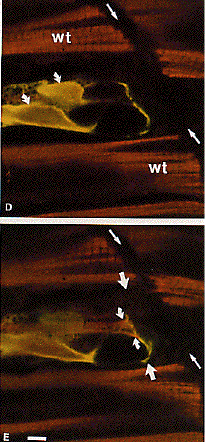
It did, however,
disrupt the attachment of the contractile apparatus to the intersomite
junction.
This disruption
was typically localized to one or the other end of the myotomal
muscle cell.
The method we
used to fix embryos, i.e. placing unanethesized embryos in formaldehyde,
leads to vigorous myotomal contractions.
We hypothesized
that the disruption of desmin organization, lead to the ripping
of myofibrils away from the weakened junction.
These studies
were the first to reveal a clear function for IFs in muscle, and
have subsequently been supported and extended by the phenotypic
analysis of desmin-null mice by Paulin, Capetanaki, and others.
|