|
13. Carriers, Pores & Pumps
|
Tweet | |||
|
|
|
Biological membranes pose a significant barrier to the movement of hydrophilic molecules. To overcome this barrier, ions and other hydrophilic molecules, such as sugars and amino acids, pass through membranes in association with various types of molecules, most of which are proteins. |
 |
| Never fear, we will discuss the basics of proteins later in the course! |
|
|
|
Hydrophobic molecules have a different problem. They are insoluble in water. To move around the body they are often bound to water soluble carrier proteins outside of the cell. They can be released into the membrane and pass through it. They can then be removed from the membrane by binding to water soluble carrier proteins in the cytoplasm. |
|
|
Molecular simulation of water through "lipid only" bilayer mambrane |
|
In fact, in the absence of aquaporins, the rate of osmotic movement of water is dramatically reduced. Aquaporins appear to be present in all organisms and to play a critical physiological role. Membrane structure and temperature: The proteins that form the channels and pumps within the membrane can, in fact, float within the plane of the membrane. |
|
This allows them to move from place to place, and to interact with one another. But to move, the membrane must be "fluid", that is the lipid molecules must be able to move more or less freely in the plane of the membrane. The molecular dynamics and behavior of a lipid bilayer changes with temperature. At low temperatures the lipid tails pack closely with one another. The energy from collisions with other molecules is not enough to knock them apart. The membrane is said to be "solid". This is analogous to a phase change (solid to liquid, liquid to vapor). Where a phase change occurs is a function of the potential energy associated with intermolecular interactions (EP), which itself is a function of how molecules interact with one another and is strongly influenced by molecular shapes, and the kinetic energy (EK) which is a function of temperature and reflects the energy available through molecular collisions. |
|
Proteins embedded in a membrane below the "melting temperature" are stuck in position. As the temperature increases, the membrane melts. The lipid tails become increasingly disordered. Membrane fluidity is critical for the functioning of many membrane proteins. |
|
|
|
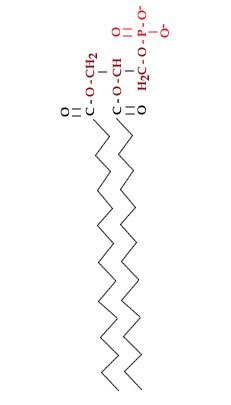
Cells can control membrane fluidity by regulating the lipid composition of the membrane. In particular, lipids can have hydrocarbon chains that are saturated or unsaturated, and the amount of cholesterol in the membrane also influences membrane melting behavior. In human cells, between 30 to 40% of the lipids, by number, are cholesterol molecules. The bulky shape of the cholesterol molecule suppresses membrane freezing. In saturated hydrocarbon chains, the carbons are linked by single bonds. The hydrocarbon chain is flexible, but more or less straight. |
|
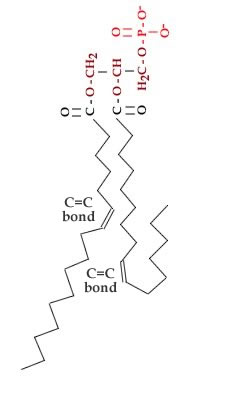
In unsaturated hydrocarbon chains, some of the carbons are linked by double-bonds. When a lipid is dehydrogenated, hydrogens are removed and -C=C- bonds are formed. The presence of a -C=C- bond leads to a kink in the hydrocarbon chain. Kinked chains cannot pack together as regularly as can straight (saturated) hydrocarbon chains. Compare the saturated fatty acid stearic acid to the unsaturated fatty acid oleic acid. |
  |
|
Both have the same number of carbons in their hydrocarbon chains. While stearic acid melts at 69°C oleic acid melts at 13°C.
|
|
|
|
Questions to answer |
|
|
Questions to ponder
|
|
|
|
|
Coupling concentration gradients: Whether or not there will be a net movement of a molecule across a membrane depends upon a number of factors. First, the molecule must be able to pass through the membrane - the membrane must be permeable to it. |
|
Second, the concentration of the molecule on one side of the membrane must be higher than the concentration on the other; such a difference in concentration between two places is known as a concentration gradient. There is, of course, an exception where "protein pumps" and coupled chemical reactions are involved, which we will discuss below. If [molecule]outside equals [molecule]inside, there will be no net movement of molecules across the membrane |
|
The system is in equilibrium, meaning that there is no net change over time and no energy is used by the system. This does not mean that the system is static; molecules are moving back and forth through the membrane, but there is no net flux. If [molecule]outside is not equal to [molecule]inside there will be a net flux of molecules from the region of higher concentration to the region of lower concentration. This flux is driven by the energy stored in the concentration gradient. Note, our analysis of net flux assumes that there are proteins present in the membrane that act as channels. There exist other classes of transporters. Co-transporters come in two "flavors", symporters and antiporters. Both transport two different types of molecules through a linked mechanism. |
|
Symporters transport two molecules together in the same direction. Examples are the Na+/galactose and Na+/I+ symporters Antiporters, such as the GlpT glycerol-phosphate/phosphate transporter moves molecules (glycerol phosphate and phosphate) in opposite directions. Using symporters or antiporters, it is possible to couple different concentration gradients, so that the flux of one type of molecule down its concentration gradient can be used to move another type of molecule up its concentration gradient. |
|
Basically, a concentration gradient of one molecule acts as a source of energy (a battery) to drive the movement of the other. If there were no membrane, or if the membrane were completely and freely permeable, this "battery" would run down very fast. |
|
|
|
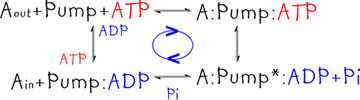
Pumping up gradients: If a membrane were completely impermeable, the concentration gradients across the membrane would remain stable. On the other hand, it would not be possible for the cell to use the energy stored in these concentration gradients. Real biological membranes are semi-permeable; they can be used both to store and access energy. The movement of different molecules across them differ based on which transport proteins are present and active. Because they are semi-permeable, biological membranes leak - without the constant addition of energy, the energy stored in concentration gradients across a membrane would dissipate over time, that is [molecule]outside will become equal to [molecule]inside. Generating and maintaining concentration gradients requires the expenditure of energy. Membrane proteins that directly use energy to generate or maintain concentration gradients are known as pumps. These are complex protein machines - some can capture energy directly from light, others use chemical energy. |
|
There are a number of molecules used to store and transfer chemical energy in biological systems. Perhaps the most important is ATP. Because ADP and phosphate are more stable than ATP + water, the ATP hydrolysis reaction is thermodynamically favorable, and can (through reaction coupling) be used to drive thermodynamically unfavorable reactions. In the ATP hydrolysis reaction (ATP + H2O to ADP + Pi), the bond between the terminal or γ (gamma) phosphate group of ATP is broken and new, more stable bonds are formed. |
|
The ATP hydrolysis reaction is coupled to the reaction that changes the structure of the pump protein; this drives a change in the structure of the pump protein and the pumping of molecules across the membrane (Ain to Aout). |
 |
|
|
|
Questions to answer |
|
|
Questions to ponder
|
|
|
|
|
[ talk amongst yourselves about the questions]
|
|
|
| replace with revised beSocratic activity |
|
|
|
revised
10-May-2014
|