| Tweet | |||
|
|
|
Organisms use energy to build complex and unstable biological molecules, maintain their structural organization (for example, maintain chemical gradients across their membranes), and to build copies of themselves. There are two sources of energy available to organisms: chemical and electromagnetic. |
 |
|
When you consider molecules, you will realize that biologically useful energy is held in molecular electron orbitals; there are no nuclear reactions going on inside cells. The structure of a molecule's electron orbitals determines the amount of energy stored in each electron. When an electron is added to a molecule the energy of the molecule increases and the molecule is said to have been "reduced". And yes, it does seem weird that adding an electron "reduces" a molecule. If an electron is removed, the molecule's energy is lowered and the molecule is said to have been "oxidized". |
|
Since electrons can neither be created nor destroyed (remember, no nuclear reactions), the reduction of one molecule is always coupled to the oxidation of another. For this reason, reactions of this type are referred to as redox reactions. There are many compounds in the natural world from which organisms can extract energy. Lithotrophs or rock eaters extract energy from inorganic compounds, such as elemental sulfur (S), molecular hydrogen (H2), and methane (CH4). Most higher organisms are either phototrophs,which extract energy from light, or chemoorganotrophs, which extract energy from organic molecules. You yourself are a chemoorganotroph. The larger the change in free energy (ΔG) associated with a reaction, the more energy is released or absorbed when the reaction occurs. |
|
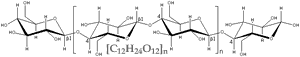
Consider wood, which is mainly composed of the carbohydrate polymer cellulose [C6H10O5]n The burning of wood can be written as a chemical reaction. cellulose + O2 ↔ nCO2 + nH2O |
 |
|
This reaction has a high negative free energy ( ΔG), it is exothermic. That means, the energy stored in the bonds of cellulose and O2 is less than that stored in the bonds of CO2 and H2O. The difference is released as heat (thermal motion, that is increased kinetic energy). |
|
|
At the same time, the activation energy of this reaction is high, which is why books do not spontaneously burst into flames. While thermodynamically favorable (because ΔG is negative), it does not occur spontaneously (at least if we use the word spontaneously as it is normally used in English). Once started, however, the energy released during the reaction acts to maintain the reaction -- the reaction becomes self-sustaining and continues until the reactants are used up. In order to be biologically useful, however, highly exothermic reactions must be controlled and the energy released must be captured in a useful form. It is hard for an organism to capture, at the molecular level, the energy of a fire. To capture energy, cells use step-by step processes. As an example, a molecule of sugar is broken down by a cell in a tightly controlled, step-by-step process known as glycolysis, from the Greek words meaning sweet (glyco) and splitting (lysis). The energy released is captured through synthesis of a number of unstable compounds, including ATP. |
|
|
|
Phototrophs, organisms that extract energy from light, capture light particles (photons) and transform their electromagnetic energy into chemical energy. Generally they store this energy in concentration gradients across membranes or in relatively unstable molecular bonds. One of the simplest methods for capturing light and transforming it into chemical energy is used by the archaea Halobacterium halobium. Halobacteria are extreme halophiles or salt-loving archaea that live in waters that contain up to 5M NaCl |
 |
|
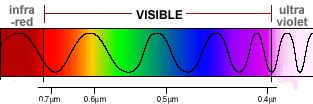
Our atmosphere allows mainly visible light from the sun to reach the earth's surface. |
|
Most biological molecules, however, do not absorb visible light. Organisms must therefore use special molecules, known as pigments, to capture visible light. |
 |
|
|
|
Light acts as both a wave and a particle (that is quantum physics for you!) The wavelength of a photon determines its color and the amount of energy it contains. A pigment's color is due to the photons it does not absorb. These are reflected and can be seen by an observer. To absorb light, H. halobium uses a membrane protein, bacteriorhodopsin. Bacteriorhodopsin consists of two components, a protein, known generically as an opsin, and a non-protein prosthetic group, the pigment retinal, a molecule derived from vitamin A. When a photon of light is absorbed by the retinal group, it undergoes a photoisomerization reaction |
|
This is a light-induced change in the molecular configuration of the pigment molecule. It leads to a change in the pigment molecule's shape and composition. This light-induced change leads to a change in the structure of the polypeptide to which the retinal group is attached. The protein itself is embedded within the plasma membrane so that the light-induced change in protein structure results in the movement of a H+ ion across the membrane and out of the cell. |
|
Because all of the bacteriorhodopsin molecules are oriented in the same way in the membrane, as light is absorbed, a H+ concentration gradient is generated across the plasma membrane. This H+ gradient is subsequently tapped by a second membrane protein, ATP synthase to synthesize ATP and water from ADP and phosphate. When the light goes off, the H+ gradient dissipates, and the light-driven synthesis of ATP synthesis slows and stops. |
|
|
|
|
|
|
Other photosystems: Cyanobacteria and plants use a more complex (homologous) system to capture and utilize light. The major pigments in this system, the chlorophylls, are based on a complex molecule, a porphyrin. |
 |
|
They absorb visible light due to the presence of a resonance structure (typically drawn as a series of single and double) carbon-carbon bonds. This actually a over-simplifies what the molecule looks like; these bonds are more like one and a half bonds (this is known as a resonance structure, but the chemistry is beyond us here). It is these pigments that give plants their green color. |
|
Chlorophyll molecules are organized into two distinct protein complexes that are embedded in membranes: these macromolecular complexes are known as the light harvesting and reaction center complexes. Light harvesting complexes act as antennas to increase the amount of light the organism can capture. This is important because photosynthetic organisms often compete with one another for light. A photon absorbed by a light harvesting complex leads to the excitation of an electron. The antennal system is highly organized and passes this excited (high energy) electron to a specialized protein-chlorophyll complex, the reaction center. From the reaction center, the high energy electron is passed to a membrane-bound, electron transport chain. |
|
As the high energy electron moves through the electron transport chain, it loses energy. Some of this energy is used to move H+s across the membrane and so generate a H+ concentration gradient, which in turn is used to break the bonds of ADP and inorganic phosphate (Pi) and generate ATP and H2O. In this cyclic reaction, a low energy electron is returned from the electron transport chain to the reaction center. |
|
|
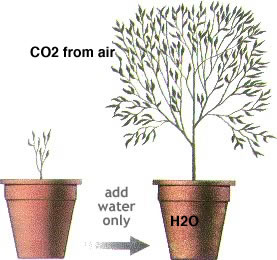
| The cyanobacteria and the green plants, developed a more complex light capturing system that could produce both ATP and a more stable form of stored energy, carbohydrate - which has the generic formula (CH20)n (carbon + water). |
|
This system uses two coupled photosystems I and II. In contrast to the cyclic system, this is a non-cyclic system in which electrons are fed into the system, excited by photons absorbed by the photosystems, and then use to reduce NADP, a phosphorylated form of the molecule NAD+, to form NADPH. The reduced NADPH is then used in a series of coupled reactions to synthesize of carbohydrate from CO2 and H2O according to the formula : 6H20 + 6CO2 + energy (NADPH) |
 |
|
Now you might be wondering, where are the electrons coming from in non-cyclic photosynthesis? During the photosynthetic process, photosystem II couple light absorption with oxidation of water molecules [link]. Four electrons, derived from two molecules of water, pass to the reaction center, 4H+s are also released and contribute to the proton gradient across the membrane. O2 is a waste product of this reaction. As we will see, O2 is essential for many organisms, particularly larger eukaryotes like us. |
|
Photosynthesis refers specifically to the reaction by which CO2 and H2O are used to synthesize carbohydrates, generally the sugar glucose. Photosynthesis is glycolysis run backward, a reaction driven by energy derived from absorbed light. ATP and NADPH are produced in the presence of light; so this part of photosynthesis is known as the light reaction. The fixation of CO2 and H2O into carbohydrate can occur in the dark, at least until the ATP and NADPH run out, and it is known as the dark reaction. |
|
|
|
|
Questions to answer
|
|
|
Questions to ponder
|
|
|
|
| replace with revised beSocratic activity |
|
|
|
revised 14-Jun-2014
|