| 9. A (very) little thermodynamics |
Tweet | |||
|
|
|
Biological systems obey the rules of chemistry and physics. They involve complex, coupled networks of chemical reactions which together produce the behavior we call life. Thermodynamic analyses tells us the extent to which a particular reaction can proceed under specific conditions. Every reaction is characterized by its equilibrium constant, Keq. Reaction kinetics tells us the rate at which the reaction actually occurs under a particular set of conditions. |
 |
|
Equilibrium is defined as the state where the concentrations of the reactants [A], [B], [C], ... and the products [Z], [Y], [X],..., remain constant over time.
|
 |
|
It is a mistake, however, to think that a system at equilibrium is static. If we were to peer into the system at the molecular level we would find that even at equilibrium, reactants will be combining to form products and products will be rearranging to form reactants. At the same time, because a system is static does not necessarily mean that it is at equilibrium. A log in the presence of molecular oxygen (O2) is not at equilibrium, even though the O2 + log ⇔ CO2+ H2O + heat reaction is highly thermodynamically favorable (more later). |
|
At equilibrium these two processes are balanced, the net flux = 0. |
|
|
| The forward reaction flux equals the back reaction flux. |
|
|
The equilibrium constant, Keq, is defined as the forward rate constant, kf divided by the backward rate constant, kr.
|
|
|
|
If, at equilibrium, a reaction has gone almost to completion, there will be very little of the reactants left and lots of the products. The product of the forward rate constant times the small reactant concentrations will equal the product of the backward rate constant times the high product concentrations. If a reaction's Keq is greater than one, there will be more product than reactant at equilibrium. |
|
If the Keq is less than one there will be more reactant than product. |
|
|
|
Coupled Reactions: Reactions can be coupled together if they share a common intermediate. In this example, the two reactions share the component "D". |
|
Let us assume that the first reaction has an Keq much less than 1, while the Keq for the second reaction is much greater than 1. What will happen? Most of the D formed by the first reaction (which is not much), will react with E (assuming E is present) and be removed from the system. This will inhibit the C+D "back reaction", while the A+B "forward reaction" will continue. More D will be produced, even though the reaction that produces it is unfavorable. |
|
|
|
Reaction rates: What does the equilibrium constant tell us about how long it will take for a reaction to reach equilibrium? The somewhat surprising answer is nothing! To understand why, consider the factors that determine the equilibrium constant and reaction rates. The rate of the reaction is determined by the molecular pathway that connects the reactants to the products. We will examine one such reaction pathway using the transport of a molecule across a membrane as an example. |
|
|
|
Consider this spatial reaction: (review video) |
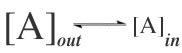 |
|
This barrier is high enough that A molecules rarely, if ever, pass through the membrane, even though the basic reaction itself is quite favorable. (What is that basic reaction?) Now, consider how adding a channel to the membrane alters the reaction kinetics. The channel "catalyzes" the reaction; it lowers the energy barrier between the two states, but is not itself used up when the reaction occurs. |
|
|
Chemical reactions are similar, although they involve the reorganization of molecules, rather than their movement from place to place. The rate of a chemical reaction is determined not by the difference in the free energy between the reactants and the products, but (to a first approximation) by the difference in free energy between the reactants and the highest energy transition state or reaction intermediate. This is the rate limiting step in the reaction. |
|
The vast majority of biological reactions require a catalyst to occur - catalysts act by reducing the free energy of the transition state. In the system we have been considering, the channel is the catalyst. Most, but not all, biological catalysts are proteins. Protein catalysts are known as enzymes. RNA catalysts are known as ribozymes. |
|
|
|
Questions to answer
|
|
|
Questions to ponder
|
|
|
|
|
|
|
| replace with revised beSocratic activity |
|
|
|
revised
10-May-2014
|