supported
by  |
|
 |
| Intermediate filaments: beginnings |
|
My work on intermediate filaments (IFs) began in the laboratory of Martin Raff (University College London). After some unsuccessful attempts at cloning neural and glial precursor cells (something that has recently lead to the discovery of neuronal stem cells and a slew of impressive papers from the Raff and other labs), in 1980 I turned to the possiblity of using the anti-IFA monoclonal antibody (developed by Rebecca Pruss), to manipulate IF organization in cultured cells. |
 |
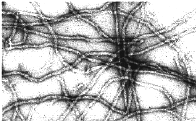 |
IFs are among the most insoluble components of the cytoskeleton. Composed of highly alpha-helical proteins, IFs can be solubilized by denaturants and reassembled, upon removal of the denaturants, into 8-10 nm diameter filaments.
|
IF assembly does not to require an external energy source, although assembly chaperones appear to be necessary in vivo. Microtubules and microfilaments are constructed of asymmetric subunits, the proteins actin and tubulin. They have an intrinsic directionality and can use the energy associated with polymerization to perform work. IF subunit proteins are also inherently asymmetric (as are all polypeptides), however the basic subunit of IF assembly is an anti-parallel (and therefore rotationally symmetric) tetramer. It is thought that assembled IFs have no inherent directionality. |
The evolution of IF proteins: IF proteins are found throughout the metazoans, but appear to be absent from unicellular eukaryotes remains unclear. The nature of the ancestral IF protein remains obscure, but could have been the lamins. The lamins form a fibrous lining on the inner surface of the nucleus. The cytoplasmic IF proteins of vertebrates differ from the lamins in the length of their helical central domain and the absence of a nuclear localization sequence. |
|
|
The central domain of all IF polypeptides is characterized by "heptad" repeats that favor the formation of helical coiled-coils. The lamins have a central domain that is 6 heptads (42 amino acids) longer than the vertebrate cytoplasmic IF proteins. Invertebrates cytoplasmic IF proteins have a lamin-like central domain. The semi-complete human genome reveals over 30 distinct IF proteins. These have been divided into 5 classes based on polymerization properties: lamins, vimentin/neurofilament-like proteins, keratins and lens proteins |
|
|
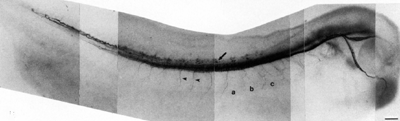 |
In contrast, the neurofilament proteins form parallel arrays that occupy much of the cytoplasmic volume of larger neurons.
|
|
PtK
cells stained in for keratin (roll over) Desmin is expressed of all types of muscle. It is associated with the Z-lines and sites of muscle-tendon and muscle-muscle cell attachment. |
|
|
Nestin, vimentin, synemin & paranemin all copolymerize with desmin.
|
1953-2004
Michael Klymkowsky and associates last updated: 24 October 2004 |