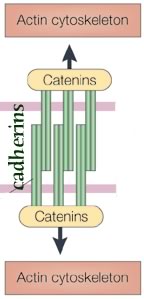
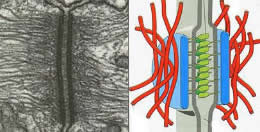
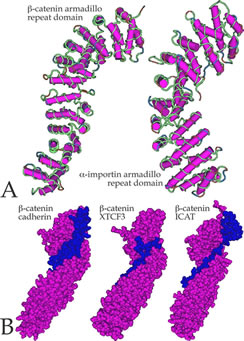
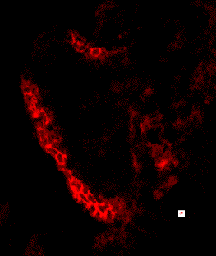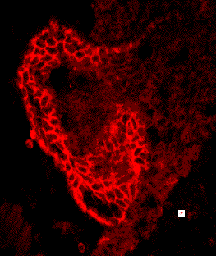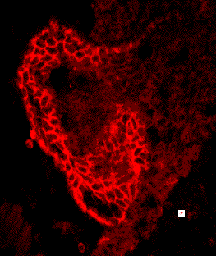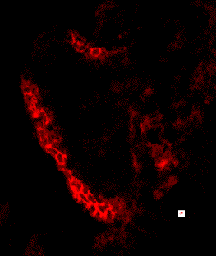
Cell shape and motility are based on cytoskeletal systems - microtubules, microfilaments and intermediate filaments and their associated proteins.
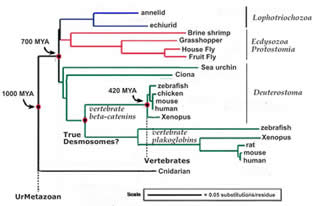
These cytoskeletal systems, particularly microfilaments and intermediate filaments are intimately associated with cell adhesion, since they are anchored, regulated by and regulate sites of cell-cell adhesion, adherens junctions and desmosomes.
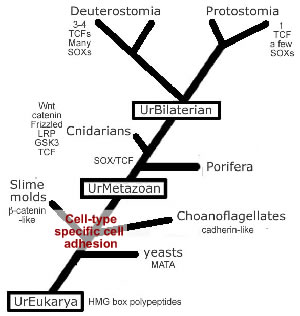
There
are a number of molecularly distinct adhesion systems. One of the
most phylogenic
ancient
are the cadherin-based adherens junctions.
The
first evidence for the emergence of a cadherin-based adhesive system
appears
within the slime molds and the choanoflagellates.